If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com




ASAHI Gaia导丝
| Brand | ASAHI(朝日) |
|---|---|
| Manufacturer | ASAHI INTECC CO.,LTD.日本朝日英达科株式会社 |
| Production Address | 日本生产地址:愛知県瀬戸巿暁町3番地100;泰国生产地址:158/1 Moo5 Bangkadi Industrial Park,Tiwanon Road,Tambol Bangkadi Amphur Muang,Pathumthani 12000,Thailand;越南生产地址:Thang Long Industrial Park,Dong Anh District,Hanoi,Vietnam |
| Intended Use | Guide wire is used for percutaneous transluminal coronary angioplasty (PTCA).导丝适用于经皮腔内冠状动脉成形术(PTCA)。 |
| References | AHW14R007S、AHW14R007P、AHW14R307S、AHW14R307P、AHW14R008S、AHW14R008P、AHW14R308S、AHW14R308P、AHW14R011S、AHW14R011P、AHW14R311S、AHW14R311P ……… |
| Manufacturer’s Website | https://medical.asahi-intecc.com/en/products-coronary/asahi-gaia.html |
| Product Brochure | |
| Registration Certificate | 国械注进20183031817 |
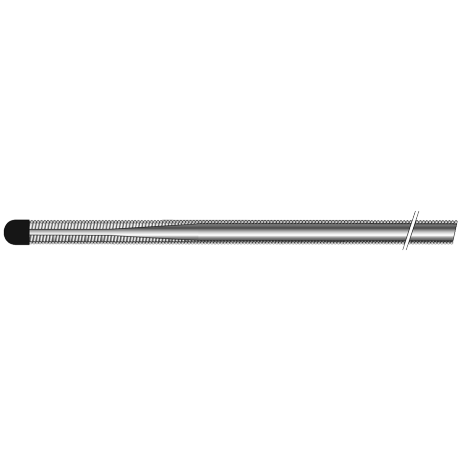
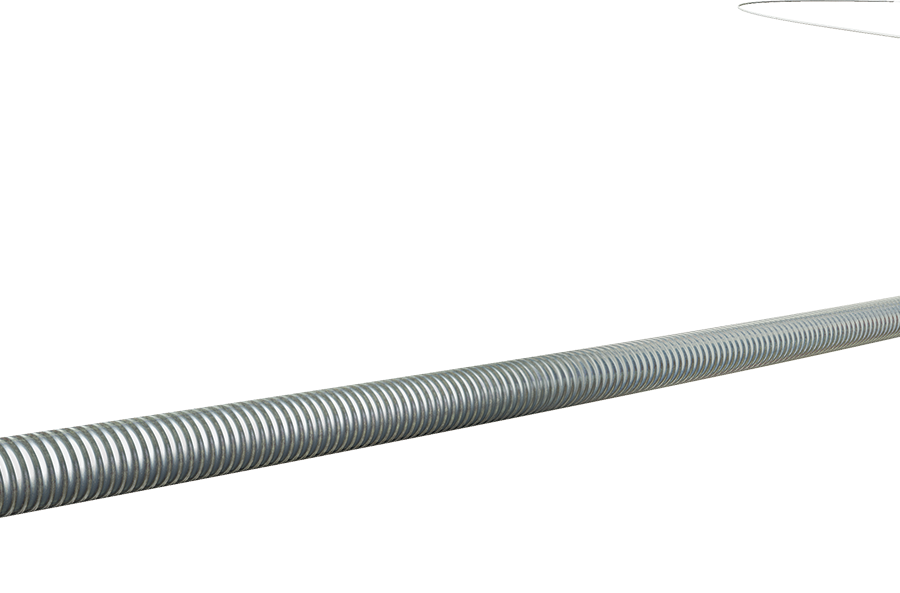
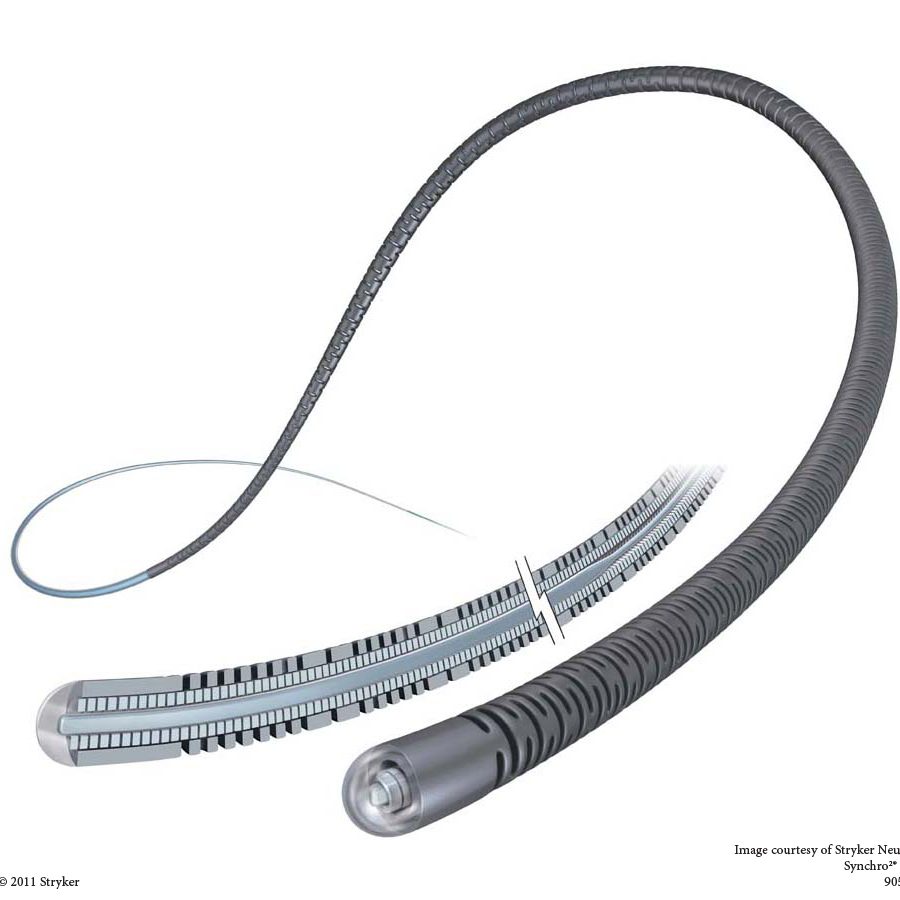
The product consists of a core wire and a coil wire, with a PTFE coating and a hydrophilic coating on the outer surface. The core wire is made of stainless steel, and the coil wire is made of platinum-nickel alloy. The product is sterilized with ethylene oxide and is for single-use only, with a sterilization shelf life of 36 months.
产品由芯丝和绕丝组成,外表面覆有PTFE涂层和亲水涂层。芯丝由不锈钢制成,绕丝由铂镍合金制成。产品经环氧乙烷灭菌,一次性使用,灭菌有效期36个月。
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Active |





Reviews
There are no reviews yet.