If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com
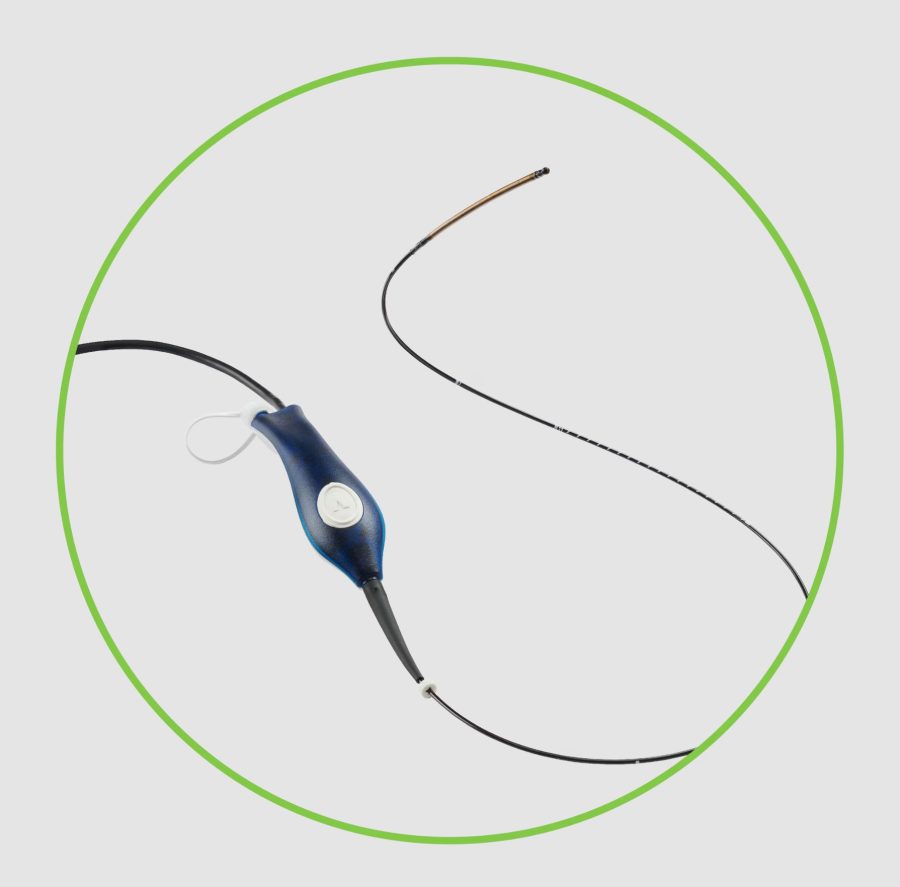
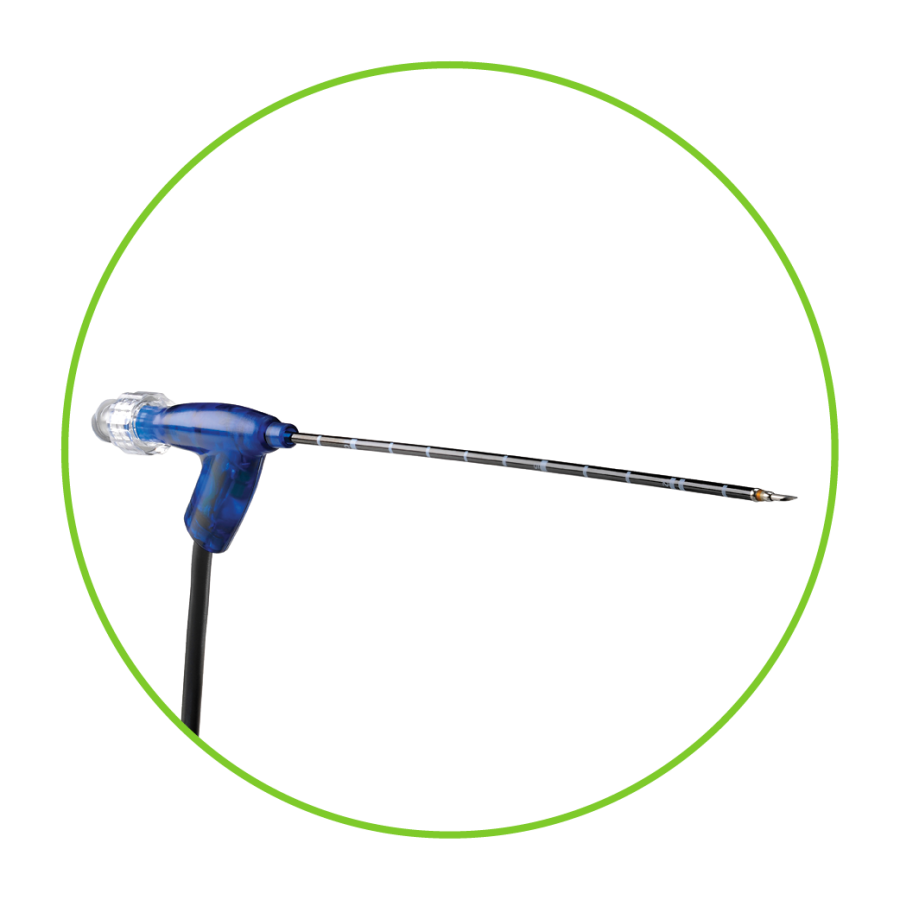

ClosureFast Endovenous Radiofrequency Ablation(RFA) Catheter静脉腔内射频闭合导管
| Brand | Medtronic(美敦力) |
|---|---|
| Manufacturer | Medtronic Inc.美敦力公司 |
| Production Address | Covidien, Boulevard Insurgentes, 19030 Libramiento, 22225 Tijuana, B.C., Mexico |
| Intended Use | This product is used in medical institutions in conjunction with the ClosureRFG radiofrequency generator (RFG2 or RFG3) for the treatment of lower extremity varicose veins (limited to superficial veins).本产品在医疗机构中与ClosureRFG射频发生器(RFG2或RFG3)联合使用,用于下肢大隐静脉曲张的治疗(限于浅静脉)。 |
| References | CF7-7-60, CF7-7-100…… |
| Manufacturer’s Website | https://global.medtronic.com/xg-en/healthcare-professionals/products/cardiovascular/superficial-vein/closurefast-rfa-system.html |
| Product Brochure | |
| Registration Certificate | 国械注进20183012473 |
The product consists of a catheter, a switch button, a Rühr adapter, a connecting cable, and a cable connector. The product is for one-time use and is sterilized with ethylene oxide.
产品由导管、开关按钮、鲁尔适配器、连接电缆、电缆连接器组成,产品为一次性使用,环氧乙烷灭菌。
Product List
| REF | GTIN | Classification Code | Description |
|---|---|---|---|
| CF7-3-60 | 00643169862944 | 01-03-04 | CF7-3-60 静脉腔内射频闭合导管 |
| CF7-7-100 | 00643169862982 | 01-03-04 | CF7-7-100 静脉腔内射频闭合导管 |
| CF7-7-60 | 00643169862968 | 01-03-04 | CF7-7-60 静脉腔内射频闭合导管 |
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Inactive |



Reviews
There are no reviews yet.