If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com
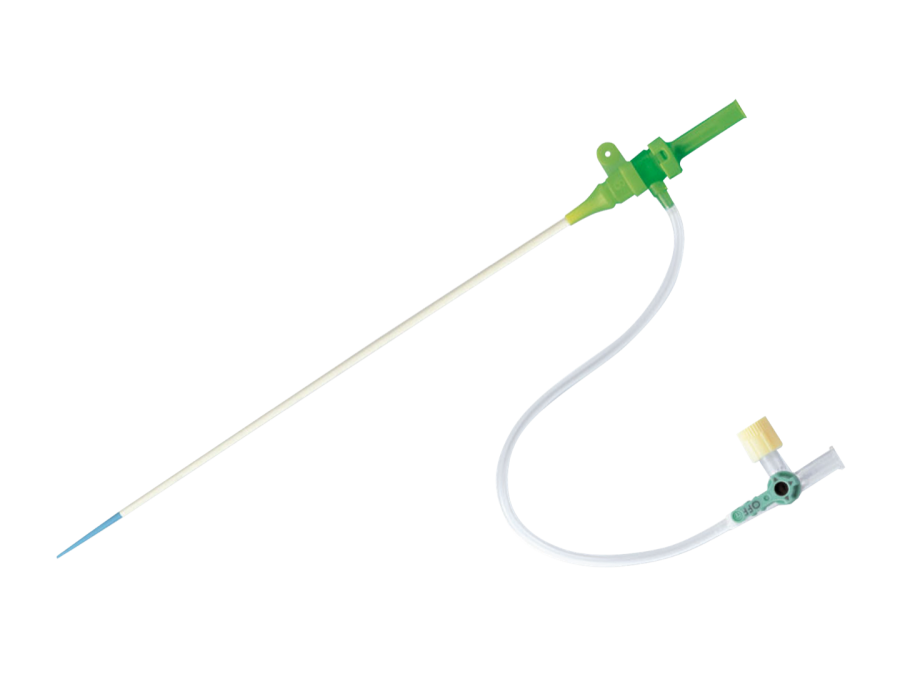
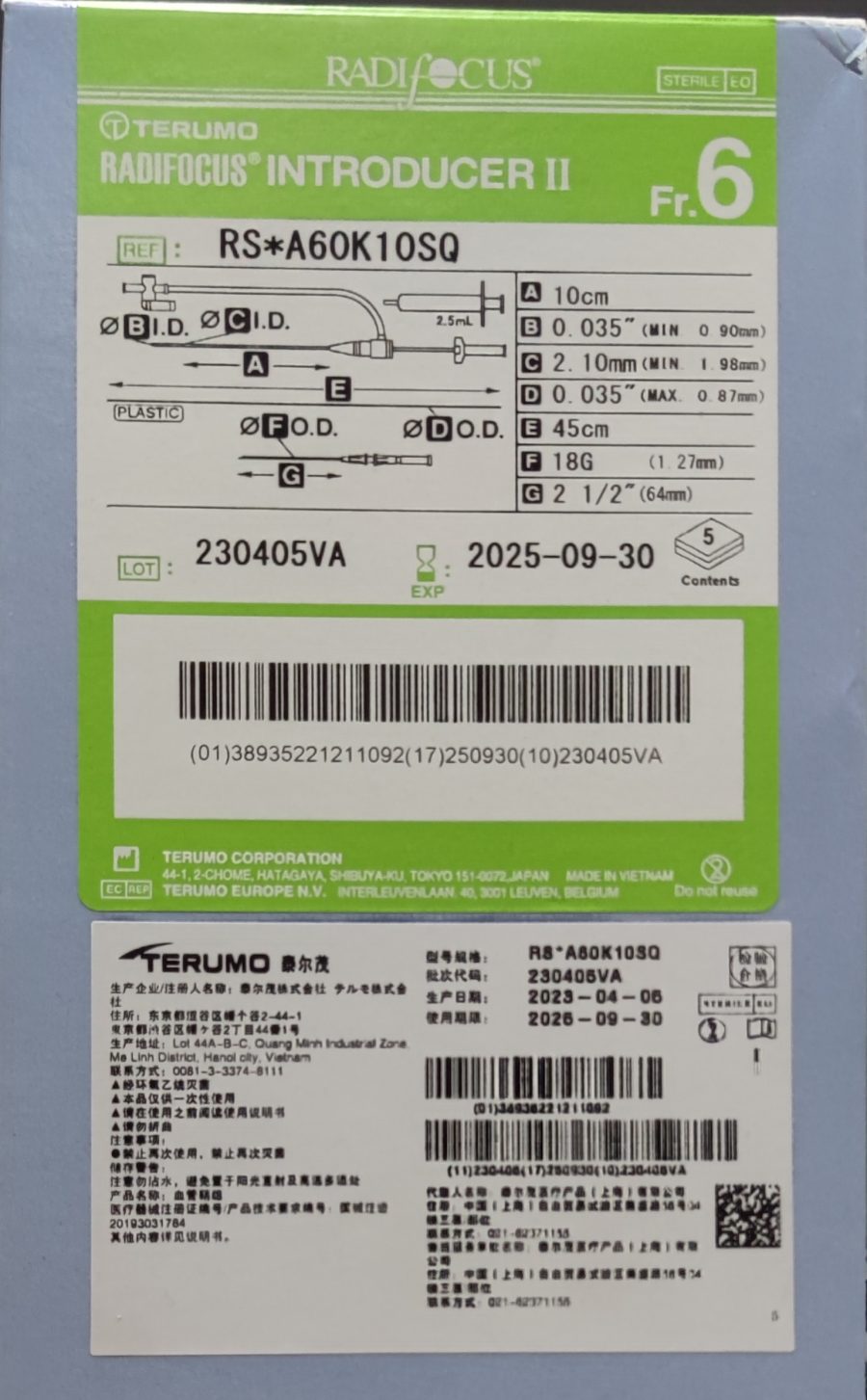
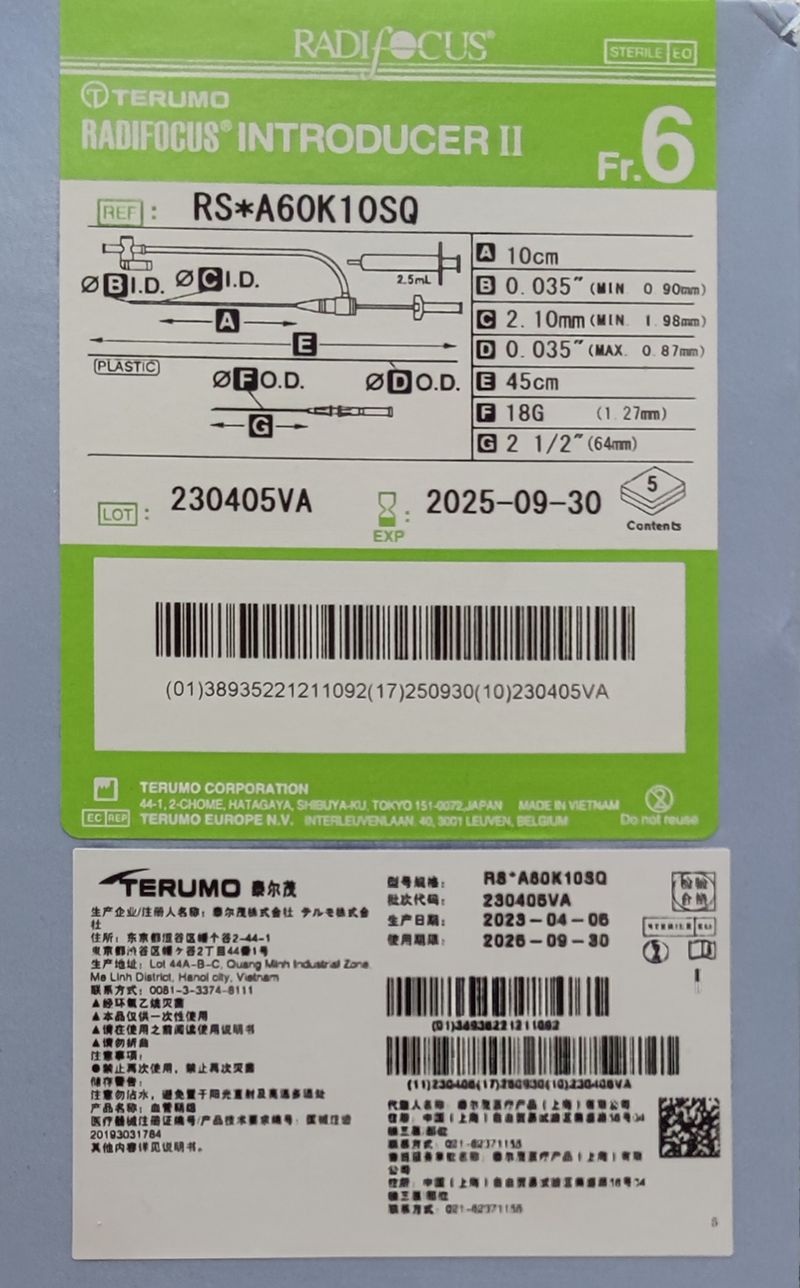
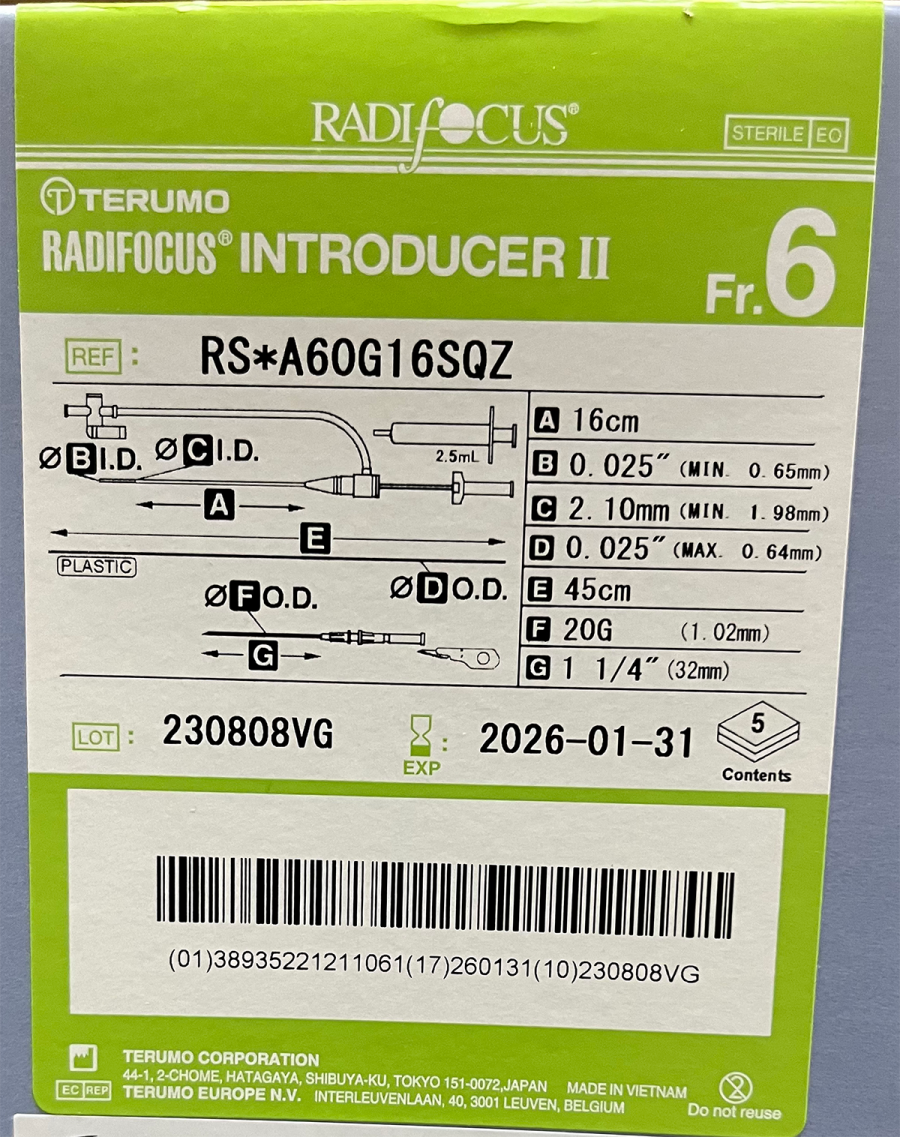
Radifocus™ Introducer II血管鞘组
| Brand | Terumo(泰尔茂) |
|---|---|
| Manufacturer | TERUMO CORPORATION (テルモ株式会社)泰尔茂株式会社 |
| Production Address | 静冈县富士宫市舞舞木町150号静岡県富士宮市舞々木町150;Lot 44A-B-C,Quang Minh Industrial Zone,Me Linh District,Hanoi city,Vietnam |
| Intended Use | This product is used for assisting in the insertion of catheters, electrodes, balloon catheters, and other instruments during interventional surgery.该产品用于在介入手术中,辅助导管、电极、球囊导管等器械的插入。 |
| References | RS*A60G16SQZ ……… |
| Manufacturer’s Website | |
| Product Brochure | |
| Registration Certificate | 国械注进20193031784 |
The product consists of a puncture needle, a guiding sheath, a catheter sheath, an expander, a guidewire, a skin incision device, and a syringe. The catheter sheath is made of ETFE containing bismuth oxide. The guiding sheath is made of ethylene-tetrafluoroethylene copolymer with 20% barium sulfate. The plastic-coated guidewire has a silicone coating on its surface. The catheter sheath can have 1-10 tungsten contrast markers within a range of 500mm from the sheath tip. The plastic-coated guidewire can have 1-10 tungsten contrast markers within a range of 300mm from the tip. It is sterilized with ethylene oxide and is for single-use only. The shelf life is 30 months.
该产品由穿刺针、导引套管、导管鞘、扩张器、导丝、皮肤切开器、注射器组成。导管鞘的材质为含氧化铋的ETFE。导引套管的材质为加硫酸钡(20%)的乙烯-四氟乙烯共聚物。塑料型导丝表面涂覆有硅涂层。导管鞘从鞘尖端起500mm的范围内,可设1-10个钨造影标记物。塑料型导丝从尖端起300mm的范围内,可设1-10个钨造影标记物。环氧乙烷灭菌,一次性使用。货架有效期为30个月。
Product List
| REF | GTIN | Classification Code | Description |
|---|---|---|---|
| RS*A10K10SQ | 34987350725111 | 03-13-14 | 血管鞘组 |
| RS*A11K10SQ | 34987350725135 | 03-13-14 | 血管鞘组 |
| RS*A40G07SQ | 38935221210880 | 03-13-14 | 血管鞘组 |
| RS*A40G10SQ | 38935221210897 | 03-13-14 | 血管鞘组 |
| RS*A40G10SQ | 34987350725173 | 03-13-14 | 血管鞘组 |
| RS*A40K10AQ | 38935221210927 | 03-13-14 | 血管鞘组 |
| RS*A40K10AQ | 34987350725210 | 03-13-14 | 血管鞘组 |
| RS*A40K10SQ | 38935221210934 | 03-13-14 | 血管鞘组 |
| RS*A40K10SQ | 34987350725234 | 03-13-14 | 血管鞘组 |
| RS*A50G07SQ | 38935221210965 | 03-13-14 | 血管鞘组 |
| RS*A50G16SQZ | 38935221210989 | 03-13-14 | 血管鞘组 |
| RS*A50G16SQZ | 34987350735271 | 03-13-14 | 血管鞘组 |
| RS*A50K10AQ | 38935221210996 | 03-13-14 | 血管鞘组 |
| RS*A50K10SQ | 38935221211009 | 03-13-14 | 血管鞘组 |
| RS*A50N25AQ | 38935221211023 | 03-13-14 | 血管鞘组 |
| RS*A50N25AQ | 34987350751134 | 03-13-14 | 血管鞘组 |
| RS*A60G07SQ | 38935221211047 | 03-13-14 | 血管鞘组 |
| RS*A60G07SQ | 34987350725395 | 03-13-14 | 血管鞘组 |
| RS*A60G16SQZ | 38935221211061 | 03-13-14 | 血管鞘组 |
| RS*A60G16SQZ | 34987350735295 | 03-13-14 | 血管鞘组 |
| RS*A60H07SQ | 34987350711701 | 03-13-14 | 血管鞘组 |
| RS*A60K10AQ | 38935221211085 | 03-13-14 | 血管鞘组 |
| RS*A60K10SQ | 38935221211092 | 03-13-14 | 血管鞘组 |
| RS*A60N25AQ | 38935221211122 | 03-13-14 | 血管鞘组 |
| RS*A60N25AQ | 34987350751158 | 03-13-14 | 血管鞘组 |
| RS*A70K10SQ | 38935221211153 | 03-13-14 | 血管鞘组 |
| RS*A70K10SQ | 34987350725494 | 03-13-14 | 血管鞘组 |
| RS*A70N25AQ | 38935221211184 | 03-13-14 | 血管鞘组 |
| RS*A70N25AQ | 34987350751172 | 03-13-14 | 血管鞘组 |
| RS*A80K10SQ | 38935221211207 | 03-13-14 | 血管鞘组 |
| RS*A80N25AQ | 38935221211214 | 03-13-14 | 血管鞘组 |
| RS*A80N25AQ | 34987350751196 | 03-13-14 | 血管鞘组 |
| RS*A90K10SQ | 34987350725555 | 03-13-14 | 血管鞘组 |
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Active |




Reviews
There are no reviews yet.