If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com
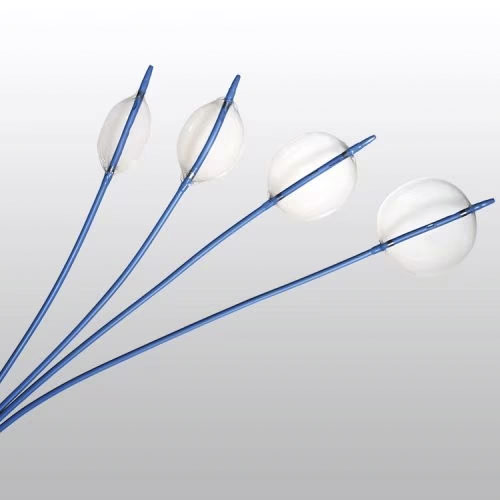
Reliant Stent Graft Balloon Catheter覆膜支架球囊导管
| Brand | Medtronic(美敦力) |
|---|---|
| Manufacturer | Medtronic Inc.美敦力公司 |
| Production Address | Parkmore Business Park West, Galway, Ireland |
| Intended Use | This product is used in conjunction with Medtronic’s self-expanding endovascular stent grafts, which are used to treat abdominal aortic aneurysms and thoracic aortic aneurysms. The device is intended to assist in the deployment of these aortic stent grafts.该产品用于与美敦力自扩张腔内主动脉覆膜支架(这些支架用于治疗腹主动脉瘤和胸主动脉瘤)联合使用。本器械可用于协助扩张这些主动脉覆膜支架。 |
| References | AB46…… |
| Manufacturer’s Website | https://global.medtronic.com/xg-en/healthcare-professionals/products/cardiovascular/aortic-stent-grafts/reliant-stent-graft-balloon-catheter.html |
| Product Brochure | |
| Registration Certificate | 国械注进20173031836 |
The product is equipped with a compliant polyurethane balloon with a maximum diameter of 46mm. The effective length of the product is 103cm. The device is designed for use with a 0.038″ or smaller diameter guidewire. The balloon features two radiopaque markers to aid in positioning before inflation. The product includes the following components: radiopaque markers, flex-resistant segment, extension tubing with a three-way stopcock, and a distal shaft. The product is sterilized with ethylene oxide and is for single-use only, with a shelf life of 2 years.
该产品配备一个最大直径为46mm的顺应性聚氨酯球囊;产品的有效长度为103cm;该产品设计使用直径为0.038”或更小直径的导丝。球囊上带有两个不透射线标记带,用于辅助球囊在扩张前的定位。产品包括以下部件:不透射线标记带,抗折段,带三通的延长管路,后端轴杆。产品环氧乙烷灭菌,一次性使用,产品有效期2年。
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Inactive |
-900x900.jpg)



Reviews
There are no reviews yet.