If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com

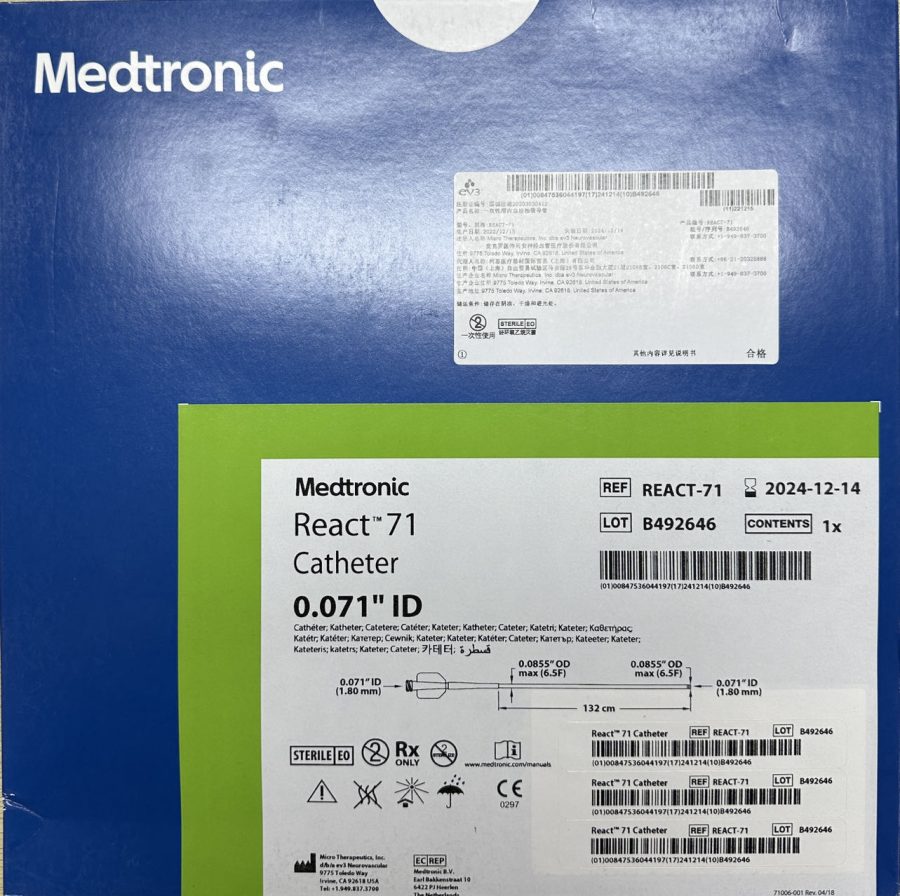
ReactTM 71 Catheter一次性颅内血栓抽吸导管
| Brand | EV3(医伟司安) |
|---|---|
| Manufacturer | Micro Therapeutics, Inc. DBA ev3 Neurovascular麦克罗医伟司安神经血管医疗股份有限公司 |
| Production Address | 9775 Toledo Way, Irvine, CA 92618, United States of America |
| Intended Use | Used for delivery of interventional devices in peripheral and intracranial neurovascular vessels. Also applicable for recanalization of large intracranial vessel occlusion (internal carotid artery, middle cerebral artery – M1 and M2 segments, basilar artery, and vertebral artery) in patients with acute ischemic stroke, within 8 hours of symptom onset. Patients who have failed intravenous tissue-type plasminogen activator (IV t-PA) or IV t-PA treatment are candidates for this therapy.用于在外周和颅内神经血管内输送介入器材。也适用于对颅内大血管阻塞(颈内动脉、大脑中动脉-M1段和M2段、基底动脉和椎动脉内)继发急性缺血性脑卒中的患者进行血管再通,而且必须在症状发作的8小时内。不能使用静脉组织型纤溶酶原激活物(IVt-PA)或IV t-PA治疗失败的患者是该治疗的人选 |
| References | REACT-71 |
| Manufacturer’s Website | https://europe.medtronic.com/xd-en/healthcare-professionals/products/neurological/revascularization-stroke/react-catheters.html |
| Product Brochure | |
| Registration Certificate | 国械注进20203030412 |
The product is a single lumen, elastic catheter made of different hardness composite materials, with a Y-shaped introducer sheath attachment. The inner layer of the catheter is made of polytetrafluoroethylene and Engage polyolefin elastomer. The catheter contains a reinforcement layer composed of coils and braided layers, made of nitinol alloy. The outer layer of the catheter includes polyamide, polyether-block-amide, and polyolefin elastomer. The distal end of the catheter has a Surmodics Serene coating and a radiopaque platinum-iridium marker. Ethylene oxide sterilization, for single-use only, with a shelf life of two years.
该产品是一根单腔、有弹性的、由不同硬度复合材料制成的导管,带有Y型导入鞘附件。导管内层材料是聚四氟乙烯和Engage聚烯烃弹性体。导管含有加固层,由线圈和编织层组成,材料是镍钛合金。导管外层材料包括聚酰胺、聚醚嵌段酰胺、聚烯烃弹性体等。导管远端带有Surmodics Serene涂层,并带有不透射线铂铱标记带。环氧乙烷灭菌,一次性使用,货架有效期两年。
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Inactive |








Reviews
There are no reviews yet.