If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com
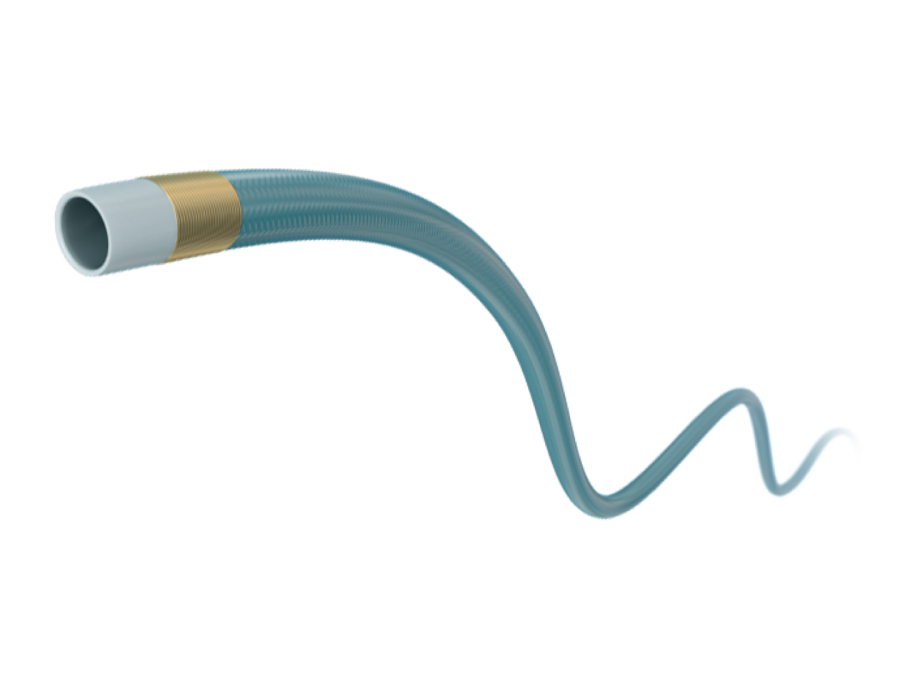


Finecross™ MG Coronary Micro-Guide catheter微导管
| Brand | Terumo(泰尔茂) |
|---|---|
| Manufacturer | TERUMO CORPORATION (テルモ株式会社)泰尔茂株式会社 |
| Production Address | Terumo Corporation Ashitaka Plant 150, Maimaigi-cho Fujinomiya City SHIZUOKA PREFECTURE (テルモ株式会社愛鷹工場 静岡県富士宮市舞々木町150) |
| Intended Use | This product is designed for patients who have difficulty in guiding wires through narrow parts of blood vessels, such as coronary arteries, after percutaneous transluminal coronary angioplasty (PTCA), to ensure the passage of wires and also for drug injection.该产品是针对经皮插入血管内之后,导丝难以通过冠状动脉等的狭窄部分的患者,在实施经皮冠状动脉成形术(PTCA)时确保导丝的通过,另外还用于注入药物。 |
| References | NC-F863A;NC-F865A……… |
| Manufacturer’s Website | https://www.terumo-europe.com/en-emea/products/finecross™-mg-coronary-micro-guide-cathether |
| Product Brochure | |
| Registration Certificate | 国械注进20163032712 |
The product consists of a guiding catheter, a hub, and a torque-resistant sheath, with a non-radiopaque gold marker and a hydrophilic coating (poly(N,N-dimethylacrylamide-co-glycerol methacrylate)). The manufacturing materials are as follows: inner layer of the guiding catheter – polytetrafluoroethylene, middle layer – polyurethane elastomer, reinforcement – stainless steel SUS304, outer layer – polyester elastomer and polyurethane elastomer; hub – nylon 12; torque-resistant sheath – polyamide elastomer. The product is ethylene oxide sterilized and has a sterilization shelf life of 24 months. It is for single-use only.
该产品由导管杆、座以及耐扭折护套组成,带有不透射线的金标记,外表面覆有亲水性涂层(二甲基丙烯酰胺-缩水甘油甲基丙烯酸酯共聚物)。制造材料为:导管杆内层:聚四氟乙烯、中层:聚氨酯弹性体、加强体:不锈钢 SUS304、外层:聚酯弹性体和聚氨酯弹性体;座:尼龙 12;耐扭折护套:聚酰胺弹性体。产品经环氧乙烷灭菌,灭菌有效期 24 个月,一次性使用。
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Active |






Reviews
There are no reviews yet.