If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com

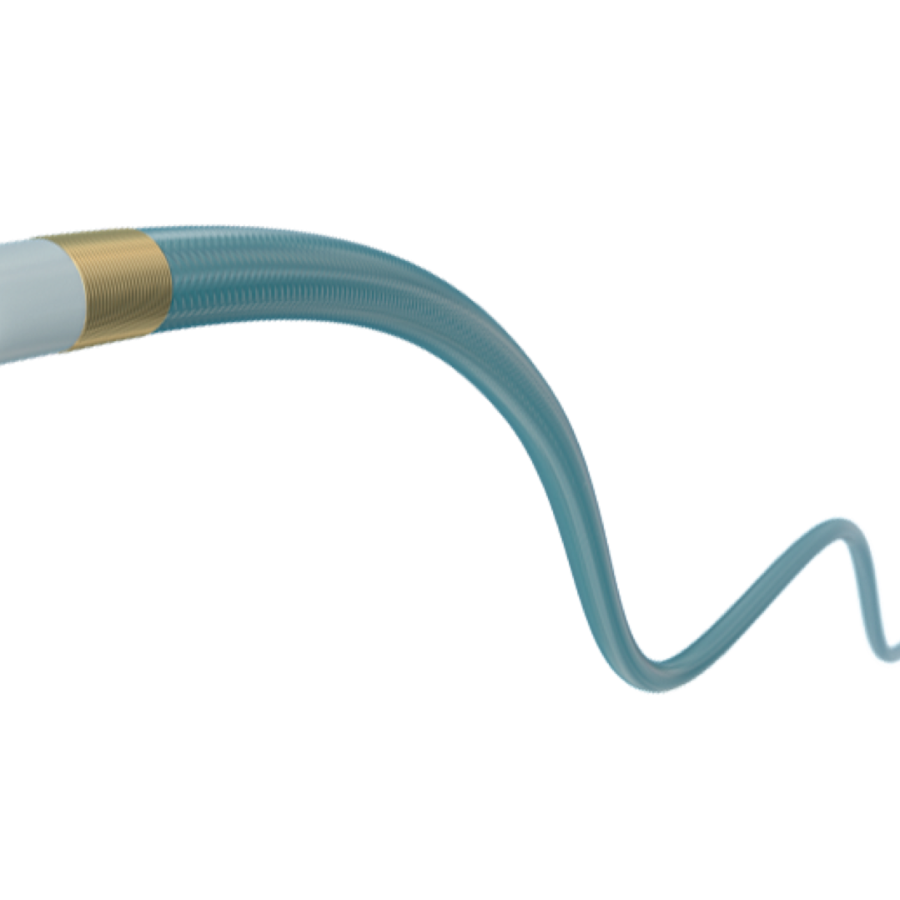
Marathon Flow Directed Micro Catheter漂浮微导管
| Brand | EV3(医伟司安) |
|---|---|
| Manufacturer | Micro Therapeutics, Inc. DBA ev3 Neurovascular麦克罗医伟司安神经血管医疗股份有限公司 |
| Production Address | 9775 Toledo Way, Irvine, CA 92618 USA |
| Intended Use | This product is suitable for professional doctors to selectively control perfusion drugs, embolic materials, and diagnostic materials such as contrast agents to peripheral and neurovascular vessels. The product is not suitable for coronary arteries.该产品适用于专业医生有选择的控制灌注药物、栓塞材料和诊断材料如造影剂到外周和神经血管。产品不适用于冠状动脉。 |
| References | 105-5056 |
| Manufacturer’s Website | https://europe.medtronic.com/xd-en/healthcare-professionals/products/neurological/avm-embolization/apollo-marathon.html |
| Product Brochure | |
| Registration Certificate | 国械注进20173036113 |
105-5056 is a single-lumen microcatheter, packaged with a steam-shaping needle. The microcatheter material is polyetherimide polymer, lined with polytetrafluoroethylene and tetrafluoroethylene. The shaping needle is made of 304V stainless steel. It is sterilized with ethylene oxide and for single use. The shelf life is 3 years.
105-5056为单腔微导管,包装内带有一根蒸汽塑形针。微导管材料为聚醚酰胺聚合物,内衬为聚四氟乙烯和四氟乙烯。塑形针采用304V不锈钢材料制成。环氧乙烷灭菌,一次性使用。货架有效期3年。
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Active |

.jpg)
Reviews
There are no reviews yet.