If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com
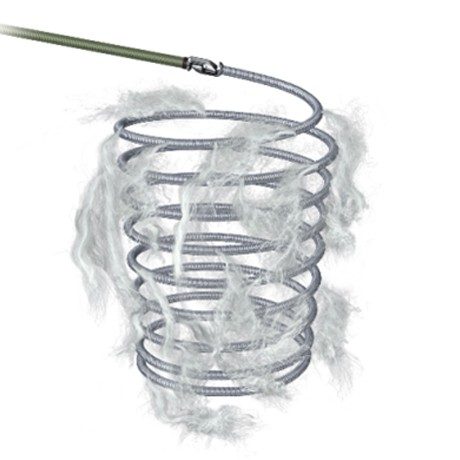
Interlock-35 Fibered IDC Occlusion System可解脱带纤维毛弹簧圈栓塞系统
| Brand | Boston(波士顿) |
|---|---|
| Manufacturer | Boston Scientific Corporation波士顿科学公司 |
| Production Address | Business and Technology Park, Model Farm Road, Cork, Ireland |
| Intended Use | This product is used to block or reduce blood flow in the peripheral vascular system during embolization procedures.该产品用于在栓塞手术中阻塞或降低外周脉管系统中的血液流速。 |
| References | M001363820, M001363610 ……… |
| Manufacturer’s Website | https://www.bostonscientific.com/en-US/products/embolization/interlock-and-idc-detachable-embolization-coils/interlock-35-fibered-idc-occlusion-system.html |
| Product Brochure | |
| Registration Certificate | 国械注进20173136219 |
The product consists of a coil, delivery wire, guiding sheath, and rotational hemostatic valve. The coil and delivery wire are connected by interlocking arms. The coil is made of a platinum-tungsten alloy and is embedded with fiber hairs. The interlocking arms at the ends of the coil are made of a platinum-iridium alloy. It is sterilized with ethylene oxide and is for single-use only. The shelf life is three years.
该产品由弹簧圈、递送丝、导引鞘和旋转止血阀组成,弹簧圈和递送丝通过互锁臂连接。弹簧圈由铂钨合金制成,嵌合有纤维毛,弹簧圈端互锁臂由铂铱合金制成。环氧乙烷灭菌,一次性使用。货架有效期三年。
Product List
| REF | GTIN | Classification Code | Description |
|---|---|---|---|
| M001363500 | 08714729793151 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363510 | 08714729797418 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363520 | 08714729792987 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363530 | 08714729795407 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363540 | 08714729793007 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363550 | 08714729793021 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363560 | 08714729793038 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363570 | 08714729797425 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363580 | 08714729793045 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363590 | 08714729793014 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363600 | 08714729793137 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363610 | 08714729793168 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363620 | 08714729793175 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363630 | 08714729792970 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363640 | 08714729793120 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363650 | 08714729793144 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363660 | 08714729797432 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363670 | 08714729795414 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363700 | 08714729792994 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363710 | 08714729793182 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363720 | 08714729793069 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363730 | 08714729793090 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363750 | 08714729797449 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363760 | 08714729793052 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363770 | 08714729795421 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363780 | 08714729797456 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363790 | 08714729793106 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363800 | 08714729795513 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363810 | 08714729793113 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363820 | 08714729795438 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363830 | 08714729795445 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363910 | 08714729795483 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363920 | 08714729795520 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363930 | 08714729796114 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363940 | 08714729795452 | 13-07-08 | Fibered IDC™ Occlusion System |
| M001363950 | 08714729795469 | 13-07-08 | Fibered IDC™ Occlusion System |
| Risk-Based Classification风险管理类别 | |
|---|---|
| Product Status产品状态 | Active |



Reviews
There are no reviews yet.