If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com
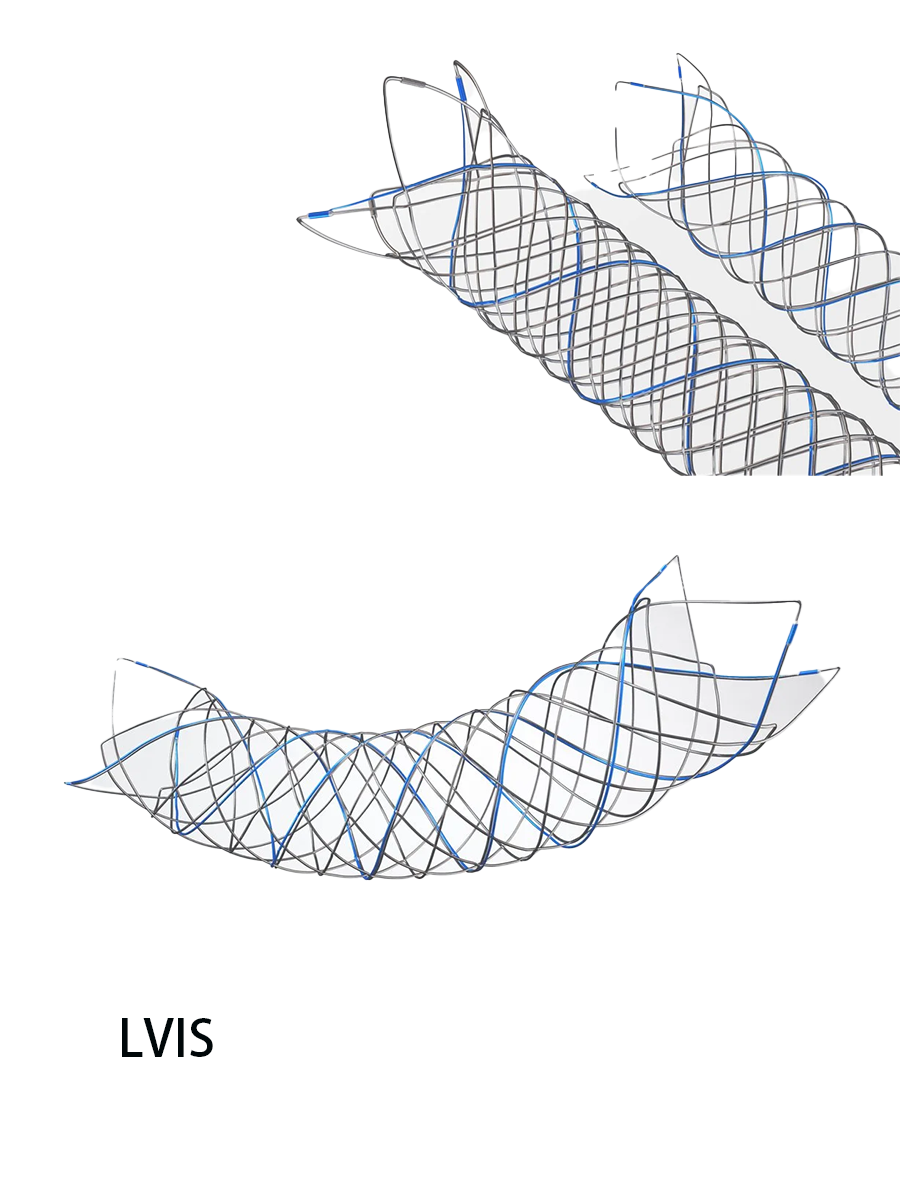
LVIS Jr. Intraluminal Support Device颅内超微支架系统
| Brand | MicroVention(美科微先) |
|---|---|
| Manufacturer | MicroVention Europe SARL美科微先欧洲有限公司 |
| Production Address | 1311 Valencia Ave. Tustin, CA 92780 United States of America; Zona Franca Coyol Alajuela Costa Rica |
| Intended Use | This product is used in conjunction with occlusion spring coils to treat intracranial cerebrovascular diseases.该产品用于与栓塞弹簧圈配合使用,治疗颅内神经血管疾病。 |
| References | 172020-CASJ ……… |
| Manufacturer’s Website | https://www.microvention.com/chapla/products/lvis-family |
| Product Brochure | |
| Registration Certificate | 国械注进20193130131 |
The system consists of a stent, a delivery wire, and an introducer sheath. The stent is made of nitinol alloy material and has three tantalum markers at each end, as well as three helical tantalum markers. The delivery wire is made of 304 stainless steel, platinum-iridium alloy, platinum-tungsten alloy, adhesive, and electrolyte formula MSC1. The introducer sheath is made of polyethylene. The product is sterilized by electron beam radiation. It is for single-use only. The shelf life is 3 years.
系统由支架、输送导丝和导入鞘管组成。其中支架为镍钛合金材料制成,两端各有3个钽标记带,并且有3根螺旋形的钽标记带;输送导丝的材料为304不锈钢、铂铱合金、铂钨合金、粘合剂、电解液配方MSC1(Electrolyte Formula MSC1);导入鞘管材料为聚乙烯。产品采用电子束辐射灭菌。产品一次性使用。货架有效期3年。
Products List
| REF | GTIN | Classification Code | Description |
|---|---|---|---|
| 172020-CASJ | 00842429103319 | 13-06-06 | 颅内超微支架系统 |
| 172010-CASJ | 00842429103296 | 13-06-06 | 颅内超微支架系统 |
| 172014-CASJ | 00842429103302 | 13-06-06 | 颅内超微支架系统 |
| 172032-CASJ | 00842429103326 | 13-06-06 | 颅内超微支架系统 |
| Risk-Based Classification风险管理类别 |
|---|


Reviews
There are no reviews yet.