If you have anyquestions
-
Call Now
-
Any Questions medrainbow.info@gmail.com
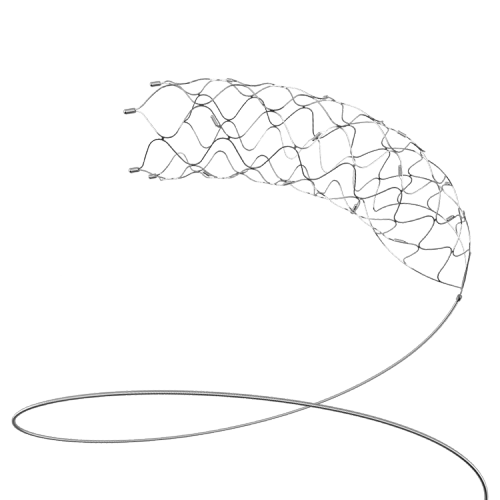

SOLITAIRE™ AB-Neurovascular Remodeling Device颅内血管支架
| Brand | EV3(医伟司安) |
|---|---|
| Manufacturer | Micro Therapeutics Inc. DBA ev3 Neurovascular麦克罗医伟司安神经血管医疗股份有限公司 |
| Production Address | 9775 Toledo Way, Irvine, CA 92618 United States America |
| Intended Use | Used for the treatment of intracranial neurovascular diseases, and used together with embolic devices for the treatment of intracranial aneurysms.适用于治疗颅内神经血管疾病,与栓塞装置一起用于治疗颅内动脉瘤。 |
| References | SAB-4-20, SAB-6-30 …… |
| Manufacturer’s Website | |
| Product Brochure | |
| Registration Certificate | 国械注进20173136562 |
The product consists of a stent, a protective sheath, and a detachable push wire. The main material of the stent is a nickel-titanium alloy, and the radiopaque marker material is a platinum-iridium alloy. Ethylene oxide sterilization, for single-use only. The product has a shelf life of 2 years.
该产品由支架、保护套和可电解脱的推送金属丝组成。支架主体材料为镍钛合金,不透射线显影点材料为铂铱合金。环氧乙烷灭菌,产品一次性使用。产品有效期2年。
Products List
| REF | GTIN | Classification Code | Description |
|---|---|---|---|
| SAB-4-15 | 00847536018396 | 13-06-06 | SAB-4-15 颅内血管支架 |
| SAB-4-15 | 00847536019829 | 13-06-06 | SAB-4-15 颅内血管支架 |
| SAB-4-20 | 00847536019843 | 13-06-06 | SAB-4-20 颅内血管支架 |
| SAB-4-20 | 00847536018419 | 13-06-06 | SAB-4-20 颅内血管支架 |
| SAB-6-20 | 00847536019867 | 13-06-06 | SAB-6-20 颅内血管支架 |
| SAB-6-20 | 00847536018433 | 13-06-06 | SAB-6-20 颅内血管支架 |
| SAB-6-30 | 00847536019881 | 13-06-06 | SAB-6-30 颅内血管支架 |
| SAB-6-30 | 00847536018457 | 13-06-06 | SAB-6-30 颅内血管支架 |
| Risk-Based Classification风险管理类别 |
|---|




Reviews
There are no reviews yet.